
Meine Kompetenz
Dr. Andreas Eberle
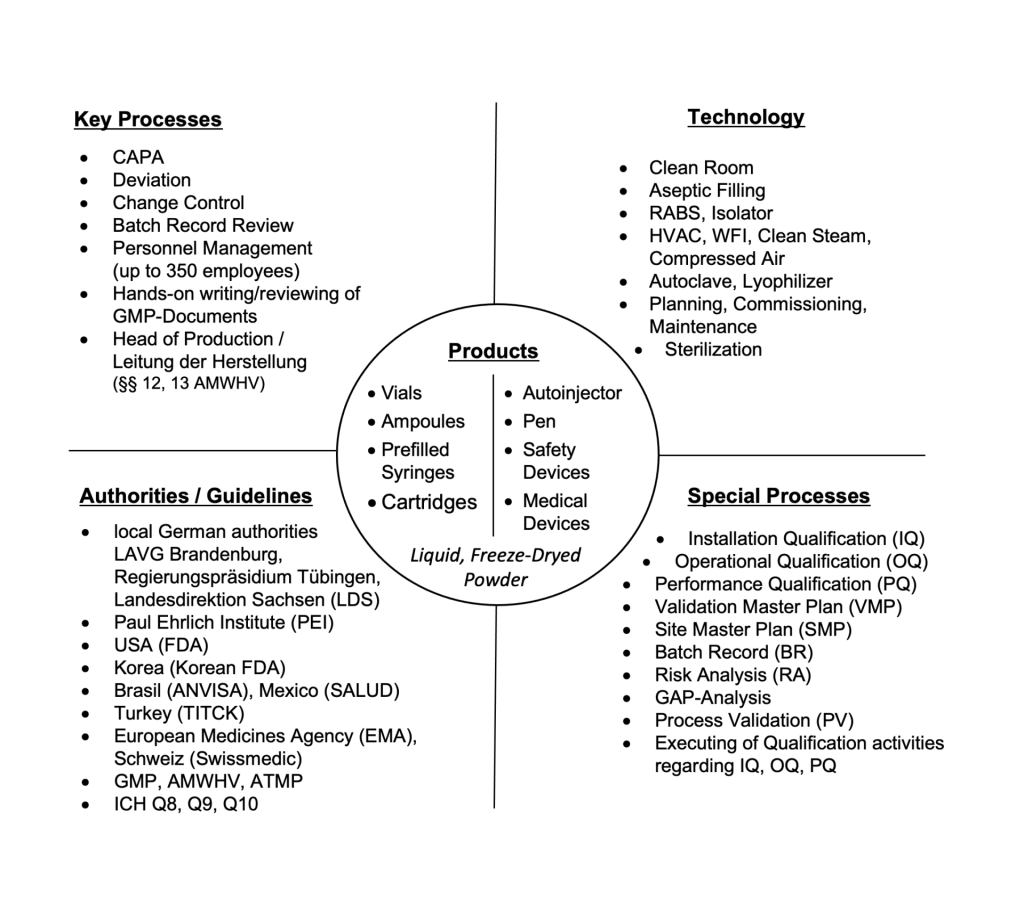
SUMMARY OF PROJECT EXPERIENCE FREELANCE
Recipharm – Wasserburger Arzneimittelwerk GmbH, Wasserburg,
GERMANY
- Coordination of preparations for the GMP-acceptance inspection of a filling line by the local authorities
Haupt Pharma Wülfing GmbH (Aenova Group), GERMANY
- Head of production (Leitung der Herstellung gemäß §12 AMWHV)
- Closing of sterile area (final APS, final Qualification of Equipment and Utilities)
Recipharm – Wasserburger Arzneimittelwerk GmbH, Wasserburg,
GERMANY
- Support Head of Operations department, reorganization of validation (APS concept, re-validation)
BellaSeno GmbH, Leipzig, GERMANY
- Consultancy for different GMP Projects, e.g. Cleanroom for 3D-print, CSV
Recipharm – Wasserburger Arzneimittelwerk GmbH, Wasserburg,
GERMANY
- Consultant QA Qualification, Upgrade-Project Annex 1, support, Support with commissioning of a isolator-based small filling line
SUMMARY OF PROJECT EXPERIENCE DURING EMPLOYMENT
ReLive Biotechnologies Ltd., Shanghai, CHINA
- Support for the establishment and expansion of production at the Shanghai site
- Support management in roadshows as part of investor relations work
CO.DON GmbH, Leipzig, GERMANY
- Planning and implementation of the Leipzig production site; 3 isolators, 16 docking stations, 144 incubators, HVAC, WFI, pure gases (compressed air, CO2-System)
- Concept development and establishment of contract manufacturing
IDT Biologika GmbH, Dessau, GERMANY
- Project responsibility for the installation of a new visual inspection area (manual and automatic optical inspection)
- Upgrade of an aseptic filling line (bulk production, filling line, pre- and postprocessing)
TEVA ratiopharm (Merckle GmbH), Blaubeuren/Ulm, GERMANY
- Modernization of an aseptic filling line for bulk syringes with associated packagingline and visual inspection (Blaubeuren/Ulm, Germany)
TEVA ratiopharm (Merckle GmbH), Kfar Saba, ISRAEL
- Subproject management: Troubleshooting of an aseptic filling system for nested
ready-to-use syringes
TEVA ratiopharm (Merckle GmbH), Mexico City, MEXICO
- Subproject management: Troubleshooting/GMP upgrade for the processing of nested ready-to-use syringes (Mexico City, Mexico)
Johnson & Johnson, Schaffhausen, SWITZERLAND
- Subproject management: New filling line for nested ready-to-use syringes, including E-beam Subproject management: Development of a new auto-injector („passive syringe“) and co-inventor of the design and introduction of a new attachable needle unit for syringes („Needle assembly for a prefilled syringe system,“ International Patent No. PCT/IB2006/002792, US No. 12/064,252) (2009)
Aventis Behring GmbH, Vienna, AUSTRIA
- Project management for qualification within the GMP upgrade of the Vienna Floridsdorf production facility (bulk manufacturing, aseptic filling, freeze-drying, supply systems)
Technical University of Berlin, Berlin, GERMANY
- Project on process development and optimization of „safety fermentation with microencapsulated systems“
- (Patent: „Microcapsules“ DE 197 56 499.2 (1997)
